PediDose: A pediatric simulated dosimetry platform for clinical use
Presentation of the problem and objective of the experiment
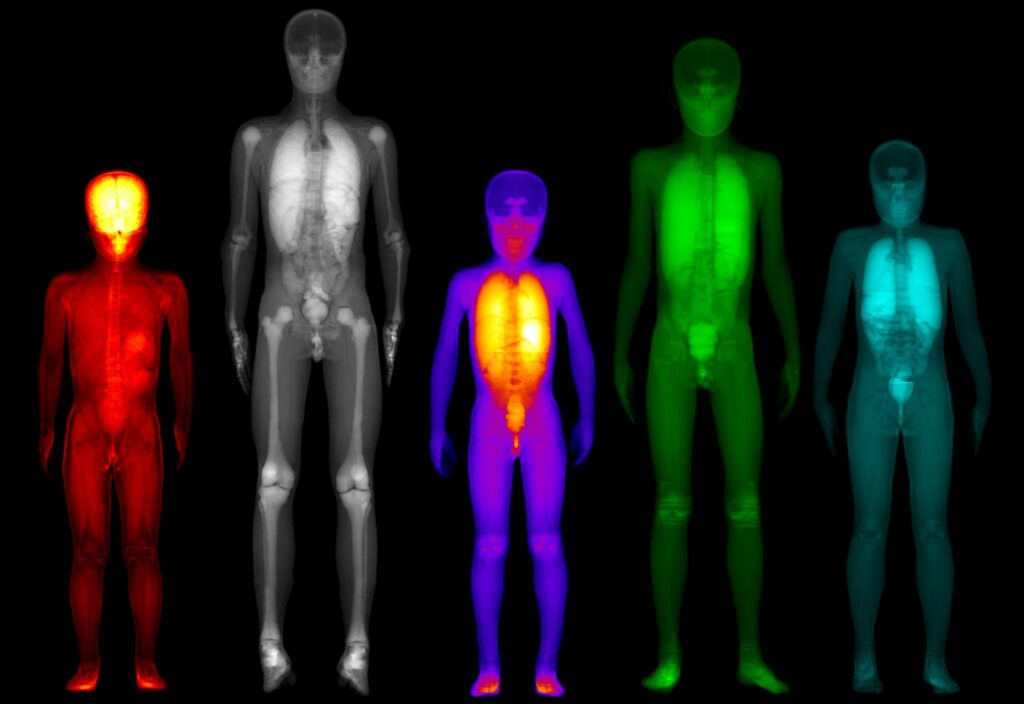
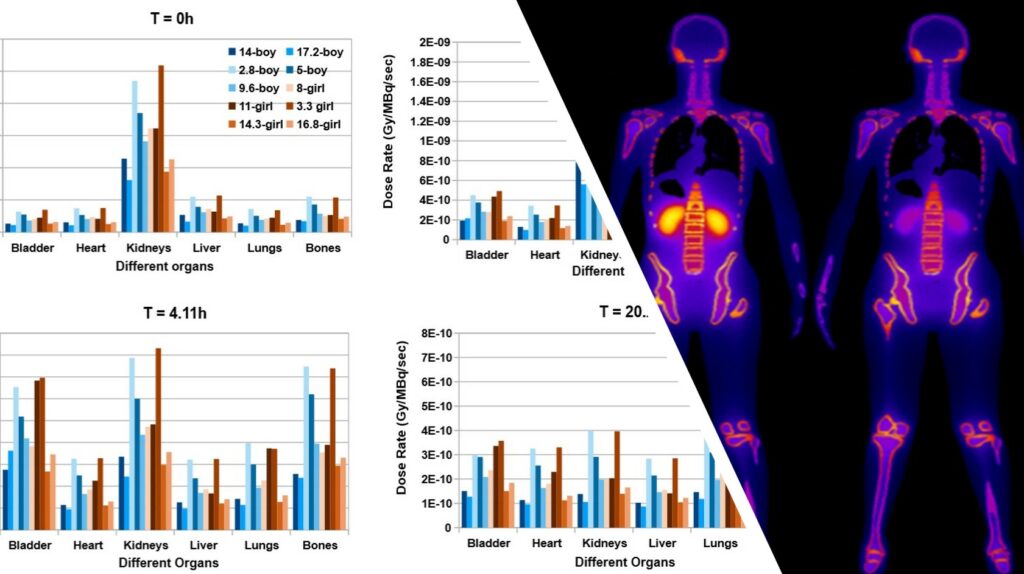
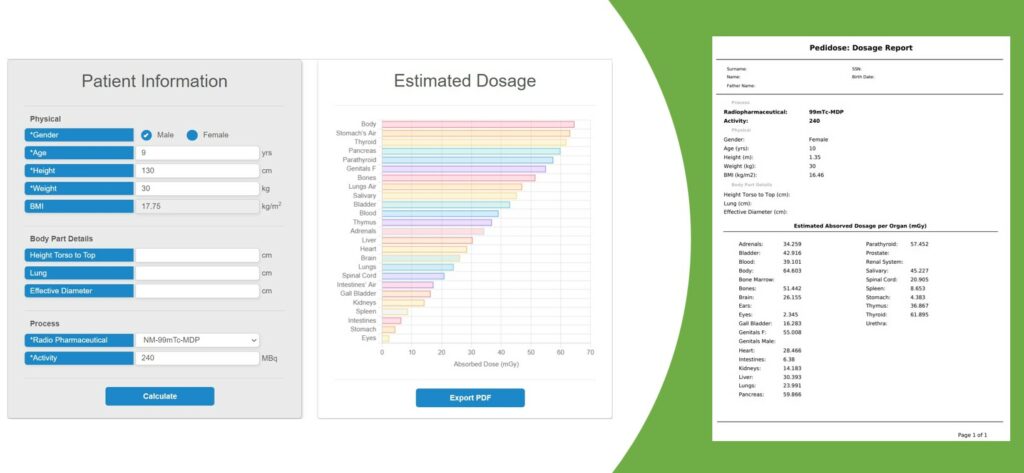
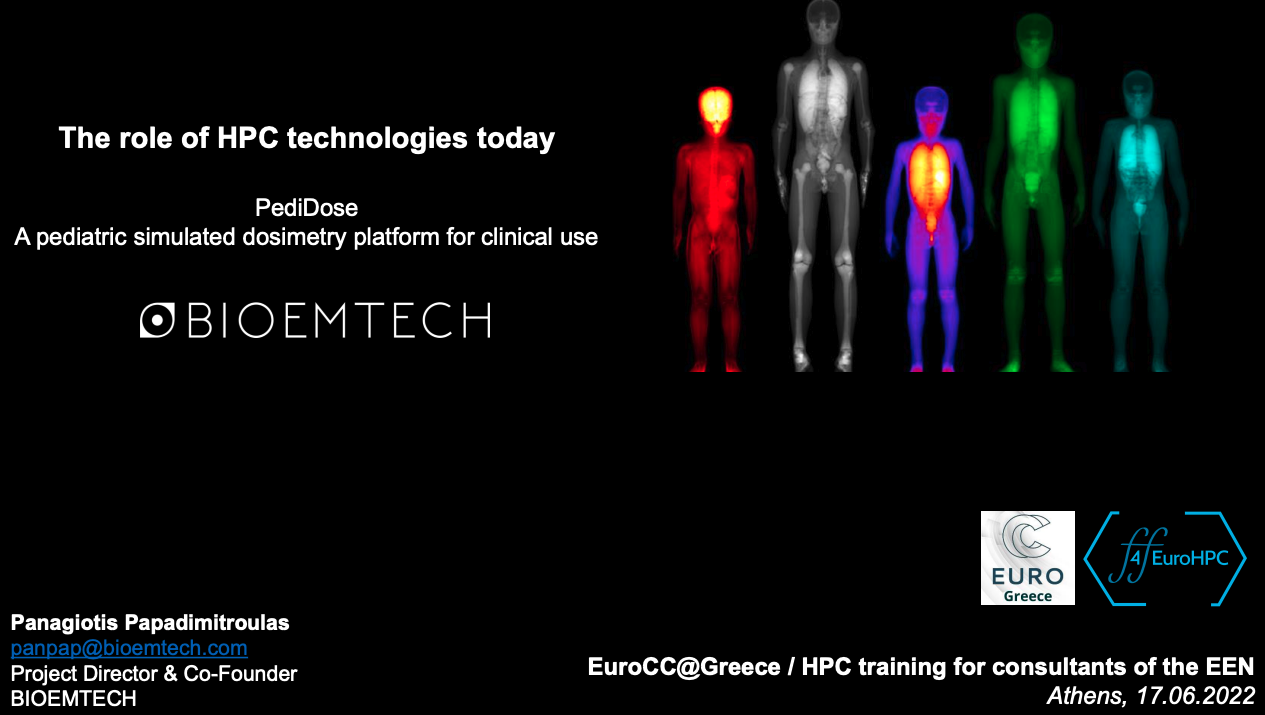
Personalized internal dosimetry is of great interest in children undergoing medical imaging examinations with ionizing irradiation. PediDose aims to exploit advanced Monte Carlo simulations, anthropomorphic computational phantoms, and Artificial Intelligence techniques to develop a realistic, simulated dosimetry database. The goal is to develop a novel software that will offer clinicians the possibility to assess internal pediatric dosimetry and optimize Nuclear Medical Imaging clinical protocols in terms of personalized prediction models.
Video Presentations
PowerPoint Presentations
Organisations involved
Short description of the experiment
MC simulations in combination with anthropomorphic digital phantoms provide accurate dosimetry estimations for clinical acquisitions (gold standard for dosimetry). Such simulations, with low statistical uncertainty, are highly intensive in terms of computational needs. A realistic simulated dosimetry database was produced using a digital pediatric population of 30 phantoms. ML algorithms were trained on the simulated data to develop a prediction dosimetry model, based on the specific anatomical characteristics of each patient. The developed tools have been integrated in a novel software product, which could be further exploited in clinical practice. PediDose brings together 2 innovative SMEs (BIOEMTECH & IKnowHow) experts in medical physics & bioinformatics and 1 HPC expert (GRNET) to efficiently implement the project.
Outlook
A beta version of PediDose GUI has been integrated to the evorad® clinical suite by IKH. Our next steps include the development of a standalone beta version of PediDose by BIOEMTECH with additional visualisation features. The goal is to extensively evaluate the novel SW in clinical environment. In parallel, both SMEs co-work on the definition of the IPs and the development of a common Marketing Strategy plan.
Lessons learned
Industrial access on HPC is necessary for advanced computational solutions, however there is the need for training personnel of HPC activities. Through PediDose BIOEMTECH explored other opportunities to utilize HPC for more company’s activities. In addition, PediDose success story showed that supporting SMEs in both funding and allocating HPC resources, in a business oriented program (FF4EuroHPC), may result in final novel products.
Expected impact
Social
PediDose offer clinicians the possibility to assess internal dosimetry and optimize pediatric protocols. Personalised dosimetry can lower administered doses and minimize radiation’s harmful effects for a very large number of treated children (sensitive group of patients). The proposed solution provides a great potential for expansion to other patient groups, utilizing the developed methodology.
Business
PediDose is expected to significantly strengthen IKH and BIOEMTECH in the EU industry of medical software and provide the SMEs with great advantages in this highly competitive area, addressing both the European and US Market. PediDose has been technically integrated into the evorad® suite, a competitive healthcare software for medical imaging (PACS) from IKH. PediDose will permit BIOEMTECH to enter the medical software market (personalised dosimetry) through business partnership with IKH extending its portfolio. Already BIOEMTECH established an autonomous SW department within the company.
Exploitation roadmap
Already both SMEs are working on the exploitation of PediDose SW. The goal is to extend and grow in the medical SW market. Currently, it is the only solution which is based on AI predictive models and the gold standard of MC simulations, which provide a competitive advantage to the existed dosimetry estimations. Already BIOEMTECH participated in the SymbIASIS project that connects hospitals and startups for clinical adaptation of innovative solutions. PediDose SW is proposed to hospitals for clinical evaluation.